Rev. Colomb. Nefrol. 2020;7(2):44-54, julio-diciembre de 2020 http://www.revistanefrologia.org
Artículo original
doi: http://dx.doi.org/10.22265/acnef.7.2.382
Barbara Leticia Dudel Mayer1
Pollyana Thays Lameira da Costa1,
Maria Elena Echevarría-Guanilo1,
Silvana Silveira Kempfer1,
Kleber Maciel da Silva Pieri2
1Graduate Nursing Program, Department of Nursing, Federal University of Santa Catarina, Florianópolis, Brazil.
2Clinical Analysis Laboratory, University Hospital, Federal University of Santa Catarina, Florianópolis, Brazil.
Introduction: Chronic hemodialysis aims to replace renal function and provides an extension of life expectancy, however, complications do occur and they can be serious even fatal. Knowing the complications can enable a better prognostic evaluation and use of an intervention approach. These can be identified when performing anamnesis and physical examination, as well as, with greater precision, by means of renal and cardiac biomarkers and imaging tests.
Objectives: to identify valid blood biomarkers to detect heart failure and kidney failure associated with kidney disease and hemodialysis.
Methods: systematic literature review conducted in August 2018 in the following: Web Of Science, PubMed, Scopus, Cinahal, Cochrane, Science Direct and Lilacs. The guiding question was: “What are the blood biomarkers used to detect heart failure and kidney failure?” A total of 537 publications were found, 94 of these appeared more than once, 383 were excluded after reading titles and abstracts, 32 were excluded after reading the full texts, and 10 were excluded in the quantitative and qualitative synthesis.
Results: 18 papers compose the final sample and report laboratory and imaging tests, instruments to assess the risk of kidney and heart failure, and also clinical management of the progression of kidney and heart failure. All the studies correlated risk of mortality and death outcome.
Conclusion: laboratory tests are important to identifying kidney and heart failure and need to be used to improve clinical management of the hemodialysis treatment of people with chronic kidney disease in order to improve quality of life and life expectancy.
Keywords: chronic renal insufficiency, renal dialysis, biomarkers, clinical alarms, diagnostic techniques and procedures (MeSH).
Introducción: la hemodiálisis crónica tiene como objetivo reemplazar la función renal y proporciona una extensión de la esperanza de vida; sin embargo, en esta ocurren complicaciones que pueden llegar a ser graves e incluso fatales, por lo que conocerlas puede permitir hacer una mejor evaluación pronóstica y establecer un enfoque de intervención adecuado. Estos factores pueden ser identificados al realizar la anamnesis y la exploración física, y, con mayor precisión, mediante biomarcadores renales, cardíacos y pruebas de imagen.
Objetivos: identificar los biomarcadores sanguíneos válidos para detectar insuficiencia cardíaca e insuficiencia renal asociada con enfermedad renal y hemodiálisis.
Materiales y métodos: se realizó una revisión sistemática de la literatura en agosto de 2018 en las bases de datos Web Of Science, PubMed, Scopus, Cinahal, Cochrane, Science Direct y Lilacs. La pregunta guía fue ¿Cuáles son los biomarcadores de sangre utilizados para detectar la insuficiencia cardíaca y la insuficiencia renal? Se encontraron 537 publicaciones, de las cuales se excluyeron 94 por estar repetidas, 383 después de leer títulos y resúmenes, 32 después de leer los textos completos y 10 en la síntesis cuantitativa y cualitativa.
Resultados: Se incluyeron 18 documentos en la muestra final, los cuales presentan pruebas de laboratorio y de imagen, instrumentos para evaluar el riesgo de insuficiencia renal y cardíaca, así como el manejo clínico de la progresión de la insuficiencia renal y cardíaca. Todos los estudios correlacionaron el riesgo de mortalidad y resultado de la muerte.
Conclusión: las pruebas de laboratorio son importantes para identificar la insuficiencia renal y cardíaca y deben utilizarse para mejorar el manejo clínico del tratamiento de hemodiálisis de personas con enfermedad renal crónica a fin de mejorar la calidad y la esperanza de vida.
Palabras clave: insuficiencia renal crónica, diálisis renal, biomarcadores, alarmas clínicas, técnicas y procedimientos de diagnóstico (DeCS).
Chronic Kidney Disease (CKD) is characterized by the gradual loss of kidney function, culminating in end-stage renal failure. The more chronic the disease, the more likely the emergence of complications-e.g., anemia, electrolyte imbalances, mineral disorders, atherosclerosis.1 These complications, in turn, trigger systemic changes, as is the case of cardiac function. Kidney disease and hemodialysis considerably increase changes in cardiac function, such as cardiomyopathy, diastolic dysfunction, congestive heart failure, coronary heart disease, and vascular changes.2,3
Even with the advancements achieved in dialysis treatment, both acute and chronic events still involve hemodialysis (HD); that is, patients resume hemodialysis if there is some complication with the remaining modalities of treatment. HD is a procedure in which a machine filters a patient's blood through a venous access to remove harmful residues in order to maintain a balance in electrolyte substances.4 HD is associated with high mortality, which is nine times higher among patients with CKD than in the general population. More than 50% of deaths are related to cardiac function. Sudden deaths are frequently caused by ventricular arrhythmias associated with HD due to sudden changes in potassium concentration or blood volume. 2 Complications happen and may be severe and fatal. Being aware of complications enables improved prognostic assessment and the employment of intervention strategies. Complications can be identified during anamnesis, physical assessment, or through kidney and cardiac biomarkers and imaging tests5
This study's objective was to identify valid blood biomarkers to detect heart failure and kidney failure associated with kidney disease and hemodialysis. This type of research can be useful in identifying studies addressing the context of biomarkers that can be used in the care provided by multidisciplinary teams to individuals with CKD undergoing HD in order to improve the quality of care delivery and intervene in the progression of disease with the purpose of preventing poor outcomes.
Systematic literature review intended to critically assess studies and collect scientific evidence of interventions used to qualify clinical practice. 6 Recommendations provided by the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (Prisma) were used.7 Studies were searched in August 2018 in the following databases: Web Of Science, PubMed, Scopus, Cinahal, Cochrane, Science Direct and Latin American and Caribbean Health Sciences Literature (LILACS).
The descriptors from the Medical Subject Headings (MeSH) were used and the search was in accordance to the following: ((«heart failure»[MeSH Terms] OR «heart failure»[All Fields] OR «heart failures»[All Fields] OR «Cardiac Failure»[All Fields] OR «Cardiac Failures»[All Fields] OR «Heart Decompensation»[All Fields] OR «Myocardial Failure»[All Fields] OR «Myocardial Failures»[All Fields] OR «Myocardial Decompensation»[All Fields] OR «Cardio-Renal Syndrome»[All Fields] OR «Cardio- Renal Syndro-mes»[All Fields] OR «Renocardiac Syndrome»[All Fields] OR «Renocardiac Syndromes»[All Fields] OR «Cardiorenal Syndrome»[All Fields] OR «Cardiorenal Syndromes»[All Fields] OR «RenoCardiac Syndro-me»[All Fields] OR «Reno-Cardiac Syndromes»[All Fields] OR «renal insufficiency»[MeSH Terms] OR «renal insufficiency»[All Fields] OR «renal insuffi-ciencies»[All Fields] OR «renal failure»[All Fields] OR «renal failures»[All Fields] OR «Kidney Insuffi-ciency»[All Fields] OR «Kidney Insufficiencies» [All Fields] OR «Kidney Failure»[All Fields] OR «Kidney Failures»[All Fields] OR «Renal Failure»[All Fields] «Renal Failures»[All Fields]) AND («blood biomarkers»[All Fields] OR «blood biomarker»[All Fields] OR «blood markers»[All Fields] OR «blood marker»[All Fields] OR «Laboratory Markers»[All Fields] OR «Laboratory Marker»[All Fields] OR «Laboratory Biomarkers»[All Fields] OR «Laboratory Biomarker»[All Fields])) AND («Renal Dialysis» [Mesh:noexp] OR «renal dialysis»[All Fields] OR «Renal Dialyses»[All Fields] OR «hemodialysis»[All Fields] OR «haemodialysis»[All Fields] OR «hemodialyses» [All Fields] OR «haemodialyses»[All Fields] OR «Extracorporeal Dialyses»[All Fields] OR «Extracor-poreal Dialysis»[All Fields]).
Studies written in any period or language, answering the following question, were used: “What are the blood biomarkers used to detect heart and kidney failure?” A total of 537 publications were identified, with 94 of these appearing more than once. The titles and abstracts of 443 publications were read and editorials, reflections, experience reports, monographs/dissertations/theses, and event abstracts, were excluded. Of these, 60 studies remained and their respective full texts were assessed. In this process, another 32 studies were excluded because they did not specifically address biomarkers of heart and kidney failure; that is, they addressed associations with other diseases in addition to kidney disease. Thus, 28 studies were taken into account in the qualitative and quantitative synthesis. Ten studies were excluded in this stage because they did not report the methodological model in such a way the study could be reproduced. Eighteen studies remained in the final sample (Figure 1).
Figure 1. Flowchart of the different stages of systematic review.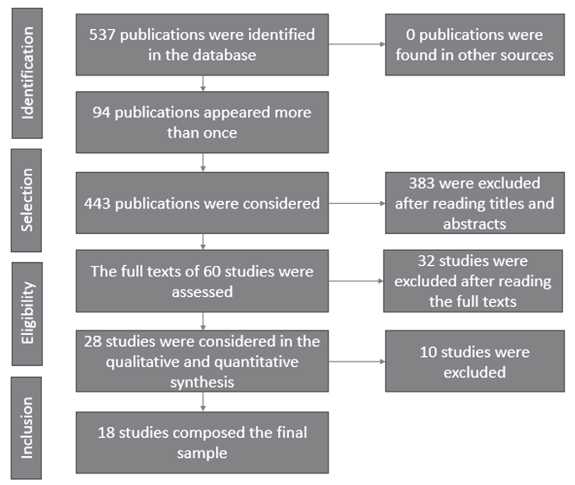
Source: own elaboration, 2019.
Level of Evidence (LE) was assessed in accordance to Oxford Centre for Evidence- Based Medicine 8 which rates the quality of evidence and strength of recommendations, to guide professional practice. Classification ranges from 1 (strong evidence, e.g., systematic reviews and metanalysis) to 5 (weak evidence, e.g., authority/expert opinion). The studies were also assessed using Strengthening the Reporting of Observational Studies in Epidemiology (Strobe), the objective of which is to assess information that needs to be included in the title, abstract, introduction, method, results and discussion of scientific observational studies. Eighteen of the 22 items assessed are common to cohort studies, case-control and sectional studies, while four items are specific to each of the three study designs. Consolidated Standards of Reporting Trials (Consort) was used to assess randomized trials, which is intended to clarify how a study was conducted, its validity and how applicable its conclusions are. For both instruments, studies that met the requirements of the Strobe's 22 items and Consort, were classified as “M” studies (Meets the requirements); as “PM” (Partially Meets the requirements); or classified “DNM” (Does Not Meet the requirements).8-10
The earliest studies were published in the 2000s and the countries of origin were the United States of America (n: 5), or were located in Europe (n: 7) or South America (n: 6). All the studies used a quantitative approach; most were cohort studies (Table 1).
Table 1. Characteristics of studies in terms of objective, design, and level of evidence.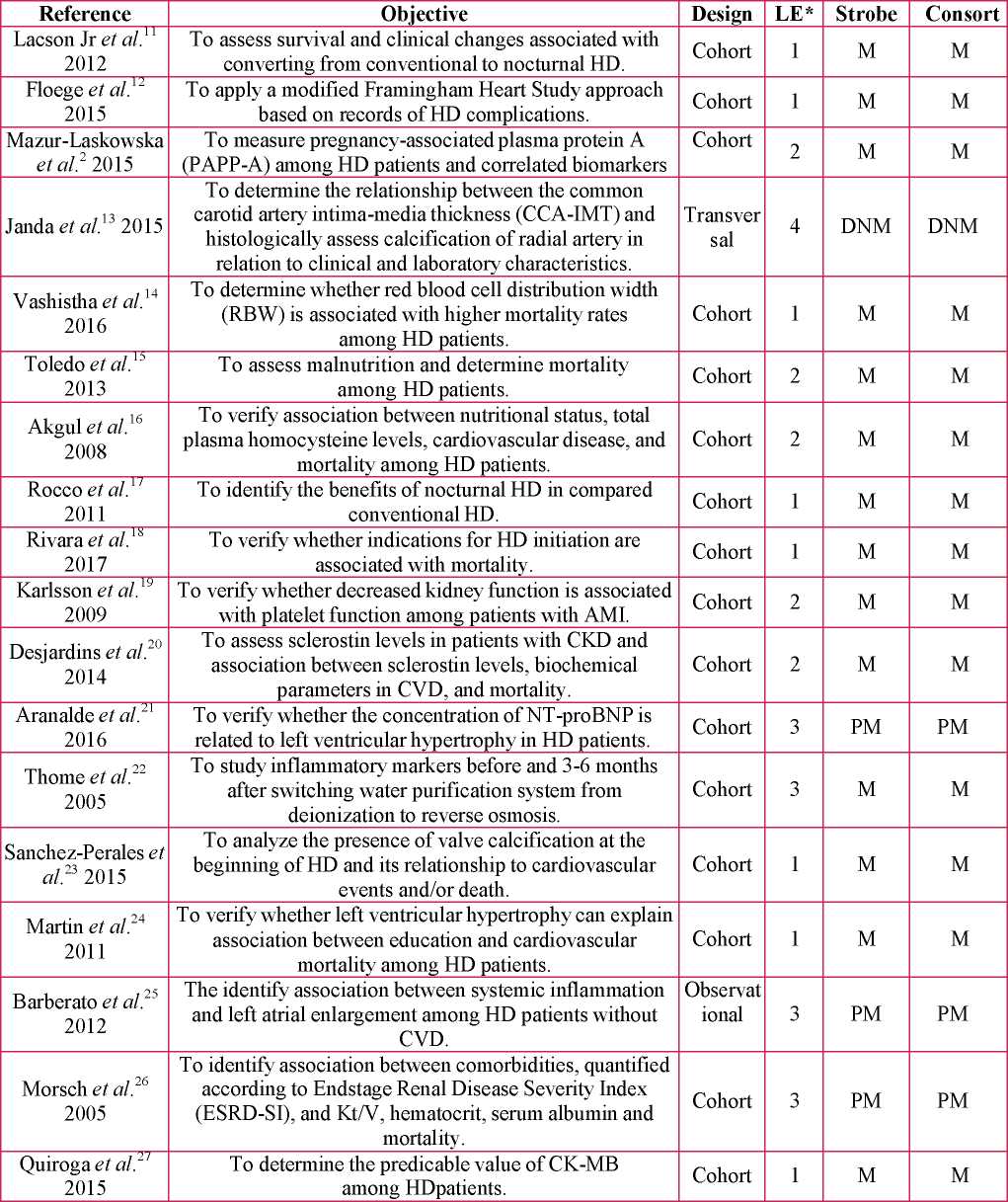
Source: own elaboration, 2019.
The blood biomarkers used in the studies to detect heart and kidney failure were identified and grouped according to biochemical, hematological, cardiac, cardiovascular, renal and inflammatory parameters that can be used in healthcare practice (Table 2).
Table 2. Details of laboratory tests to identify heart and kidney failure, 2019.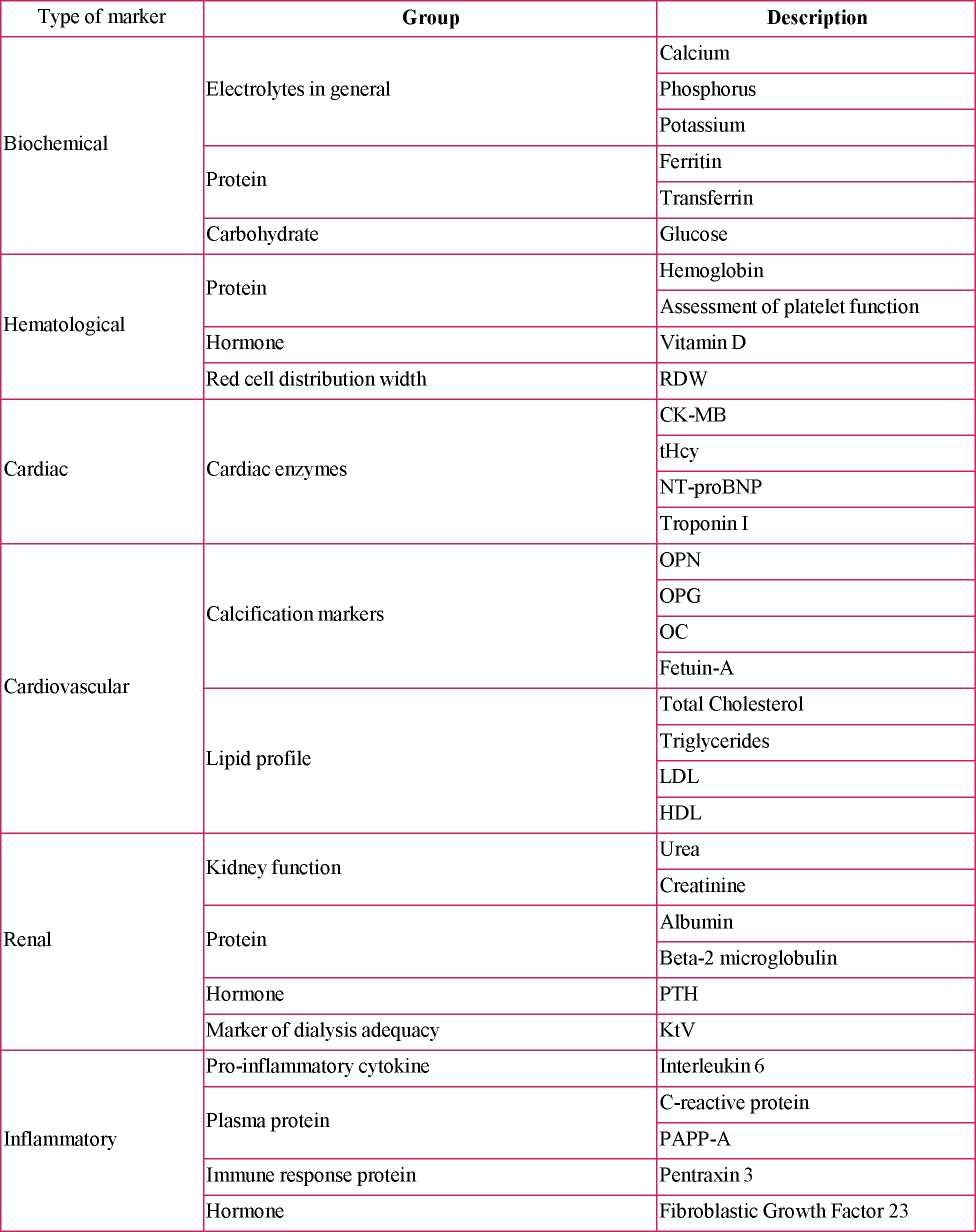
Source: own elaboration, 2019.
Imaging tests included Doppler echocardiography, electrocardiogram, ultrasound imaging of the carotid artery and von Kossa's method, used in histology to detect the presence of calcium in the radial artery. The studies also addressed previous diseases, sociodemographic factors, diabetes mellitus, cardiovascular risk factors, coronary heart disease, stroke, atrial fibrillation, acute myocardial infarction, cancer, and acute lung edema, which were correlated with a risk of mortality and mortality.
Laboratory tests include PAPP-A, which was measured in 78 patients for 60 months and correlated to HD routine tests. The median of PAPP-A levels was two times higher than the upper reference limit (140mIU/L), while values 14 times higher were found. Patients with CKD with cardiovascular events presented higher PAPP-A levels, indicating significant damage to endothelial cells and consequent increased risk of death. This same test was positively associated with serum sodium, potassium, and NT-proBNP.2,28,29
RDW (iron-deficiency anemia marker), can be used as a marker of nutritional deficiency, inflammation and to predict mortality. 30-32 One study addressing 109,675 patients reports RDW percentages between 14.5% and 17.5%. Association was found between RDW and mortality risk, that is, greater RDW levels were associated with an increased risk of death over time. The pathogenesis of high RDW levels is complex and some hypotheses include a inflammatory process that results from HD, which inhibits bone marrow function, changes iron metabolism, inhibits erythrocyte maturation and promotes oxidative stress leading to increased red blood cell heterogeneity.14,33,34 The nutritional state of a patient is directly related to hematological changes. One study assessed malnutrition based on three systems: Wolfson, Beberashvili and the International Society of Renal Nutrition and Metabolism (ISRNM). These systems serve to measure nutritional status and predict mortality. ISRNM predicted a fourfold higher risk of death. During the study, 21.5% of the participants died due to malnutrition15 Association between malnutrition, inflammation, and comorbidities is one of the reasons for poor outcomes. In this context, one study sought to identify association between nutritional status, total plasma homocysteine (tHcy) levels, cardiovascular disease (CVD), and mortality among 124 patients undergoing HD for two years. The level of tHcy was higher among patients who died due to CVD (8.8%). A positive relationship was found between tHcy and albumin and creatinine16,35
Another laboratory text, sclerostin, was assessed in Cases of histological arterial calcification were also association with vascular disease markers and mortality in 140 patients. High sclerostin levels are correlated with inflammation markers, phosphate, fibroblast growth factor, and arterial stiffness. Mortality was present regardless of age and inflammation parameters. 18 Cardiac events were assessed in 211 patients, considering the cardiac biomarker creatine kinase-MB (CK-MB). The level of CK-MB was 1-2ng/mL, which does not exceed normal laboratory parameters. When it wascorrelated with other variables to predict cardiovascular events (such as age, sex, other chronic diseases), there was an increase of 17% in the discrimination of risk 27 Cardiovascular condition was assessed using platelet aggregation during the course of myocardial infarction (AMI) among patients with and without a CKD diagnosis. The study addressing 413 patients with AMI hospitalized in a cardiac intensive care unit presented the following predictors: TFG below 60ml/ min/1.73m2, comorbidities, medications, and inflammation markers and hemostasis. There was significant increase in platelet aggregation in the first three days of hospitalization, regardless of kidney function. This occurs more abruptly in patients with TFG below 60ml/min/ 1.73m2. Advanced age, elevated plasma fibrinogen and diabetes mellitus were associated with platelet aggregation19,36,37
One study's objective was to identify the relationship between left ventricular hypertrophy (LVH), mortality and low education level.24 A total of 113 patients, assigned to two groups, participated in the study: up to three years of schooling and four or more years of schooling. A difference of 5.5 years was found regarding cardiovascular mortality when comparing educational levels. LVH was associated with Creactive protein, and cardiovascular mortality, as well as creatinine, systemic arterial hypertension, and different educational levels. LVH and risk of mortality was verified using NT-proBNP, cardiovascular failure and hypertrophy markers in a group of adult HD patients. In addition to the laboratory tests, echocardiograms were performed to measure ventricular mass. A highly significant relationship was found between NT- proBNP and LVH; the latter can be a useful biomarker of ventricular mass. A value greater than 10,000pg/ml can identify HD atients with an increased risk of death. 21,38,39
Cases of histological arterial calcification were also verified. The relationship between common carotid artery, intima-media thickness (CCA-IMT) and histological calcification of radial artery in relation to clinical characteristics and laboratory markers, was verified 13 One study with 59 patients identified significant correlation between CCA-IMT with glucose blood tests, osteoprotegerin and pentraxian 3. Calcification of the radial artery was found in 34 patients who also presented high CCA-IMT. Higher CCA-IMT was associated with more advanced calcifications. The presence of common carotid artery was a positive predictor of calcification of the radial artery, while calcification of the radial artery was a significant predictor of mortality. One study assessed valve calcification in 256 patients and associated it with demographic factors and cardiovascular risk 23,40 Acute myocardial infarction, stroke and death occurred in 26% of the patients; advanced age, coronary disease, and stroke predicted cardiovascular events. Association between systemic inflammation and left atrial dilatation was found in 58 patients through PCR measurement, interleukin 6 and Doppler echocardiography; high PCR was related to left atrial enlargement. Arteriosclerosis is a major complication of CKD and micro-inflammation is involved in atherogenesis, associated with uremia and dialysis water 25,41 Therefore, the study verifying dialysis water of two purification systems - deionization and reverse osmosis - addressing 47 patients, showed that uremic cases presented a decrease in PCR levels when water purified via reverse osmosis was used in dialysis, inducing fewer inflammatory processes and lower atherosclerosis. Sixteen of the participants died. 22
The methods to assess the risk of developing and progressing kidney and heart failure include: endstage renal disease severity index (ESRD-SI); Dialysis Outcomes and Practice Patterns Study (DOPPS); European Incident HD Patient Database (AROii); modified Framingham Heart Study approach; and risk of adverse events at the beginning of HD. These were correlated to indicators, laboratory and imaging tests, body mass index, smoking, glomerulonephritis, renal cysts, vascular access, HD complications, and death.
One of the aspects analyzed included indication for HD initiation and mortality. Indications for initiating therapy included laboratory evidence, uremic symptoms, volume overload, and hypertension. Of 461 patients, 183 (40%) died within 2.4 years, on average. After relating HD initiation to demographic variables, coexisting diseases, and glomerular filtration rate, the risk of mortality was 1.69 times greater. 18
The Endstage Renal Disease Severity Index (ESRD-SI) was used by one study to verify association between comorbidities, Kt/V, hematocrit, serum albumin, and mortality. The odds ratio of 40 patients for each ESRD-SI point was 10%; the factors with the greatest impact on mortality were diabetes mellitus, CVD and bone diseases.26,42,43
Researchers applied a modified approach of the Framingham Heart Study to verify 1- and 2-year mortality rates. They used two European databases. A mortality rate of 13/100 patients/year was found. Increased age, low body mass index, CVD, cancer, venous access catheter, laboratory tests with abnormal results, were identified as predictors of mortality. 12,44
Nocturnal dialysis was presented in two studies correlating sociodemographic factors, comorbidities and preexisting chronic diseases, laboratory and imaging tests, and dialysis indicators, such as the number of sessions per week, length of sessions, ultra filtrate, and KtV.
One study reports that improved HD results depend on the length of sessions. Thus, the researchers addressed 746 patients undergoing nocturnal HD and 2,062 patients undergoing conventional HD. The mortality rate was lower (19%) among patients undergoing nocturnal HD compared to those undergoing conventional HD (27%). Mortality decreased 25% among patients undergoing nocturnal HD when associated with age, body mass index, length of dialysis, increased interdialytic weight gain, albumin, hemoglobin, dialysis dosage, calcium, decreased pre-dialysis systemic blood pressure, ultrafiltrate rate, phosphorus, and leucocyte count 9,45-48 Therefore, the study reports that nocturnal HD has favorable clinical features, laboratory biomarkers, and improved survival compared to conventional HD. Although one study addressing 87 patients undergoing conventional HD (n: 42) and nocturnal HD (n: 45) does not report any correlation between different HD modalities and mortality rates, there was an increase in vascular access events, improved control of hyperphosphatemia and systemic arterial hypertension in the case of nocturnal HD 17,49,50
This systematic literature review was intended to identify valid blood biomarkers to detect heart and kidney failure associated with kidney disease and hemodialysis treatment. This review shows that laboratory tests aid the identification of kidney and heart failure among HD patients with CKD. All the studies reported deaths associated with failure of these systems. The studies employed a quantitative approach and were conducted on three continents, in more than 10 different countries. This review's results present the most diverse possibilities of detecting kidney and heart failure and, for this reason, can support the planning of treatment of people with CKD with greater life expectancy and improved quality of life. Preventable complications that lead to kidney and heart disorders and death need to be predicted so that measures can be implemented.
There are none
There are none
Present work happens with the support of the Coordination of Improvement of Higher Education Personnel (CAPES) - Brazil.